Effect of biological treatment in uncontrolled severe chronic rhinosinusitis with nasal polyps in Belgium: a multicentre real-world data study
Introduction
The availability of biologicals for severe uncontrolled chronic Rrinosinusitis with nasal polyps (CRSwNP) has revolutionised the therapeutic landscape of nasal polyp patients, with biologicals being included in the latest international guidelines for patients with CRSwNP (1-4). Omalizumab, mepolizumab and dupilumab are registered and reimbursed for the indication of severe uncontrolled CRSwNP in several countries worldwide (5,6). The efficacy and safety of these biologicals have been demonstrated in large phase III double blinded placebo controlled randomized trials (DBRCT) and several indirect comparison studies have tried to compare the efficacy of those different biologicals (7-13).
However, due to the heterogenicity of the inclusion criteria and treatment outcomes in the different trials, a true comparison is impossible, highlighting the need for head-to-head comparison trials and real-world efficacy (RWE) registries. To date, RWE registries have mainly studied dupilumab in Italy (14), the Netherlands (15), Canada (16) and Germany (17). Only limited, mostly retrospective, data are available on mepolizumab and omalizumab (18,19). As these two biologicals were the first to be approved and reimbursed in Belgium (20), a multicentre RWE study was initiated across eight rhinology centres in Belgium. The study focused on the RWE of both biologicals in the first wave of patients with uncontrolled CRSwNP, treated for six months with biological therapy for uncontrolled CRSwNP, that met the national reimbursement criteria. In addition to studying the RWE of these biologicals, this study also addresses the impact of biological therapies on the recently updated definition of control in CRSwNP by EUFOREA/EPOS, defined as an absence of clinically relevant sinonasal symptoms of active disease (21). As biologicals are thought to possibly have a disease-modifying potential (22), understanding their effect on long-term disease control without sings of active disease, also defined as remission (21), is of strategic importance for both patients as well as physicians.
Materials and methods
Patient population
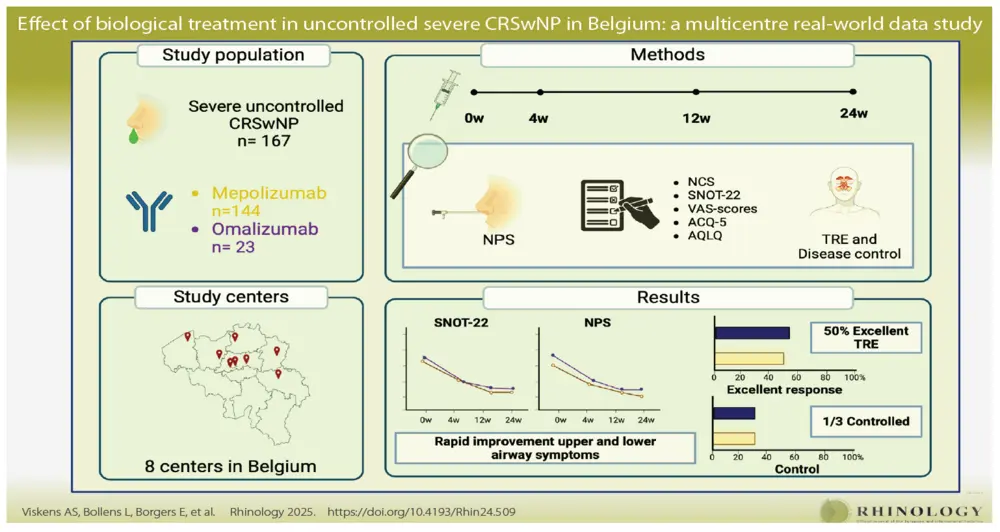
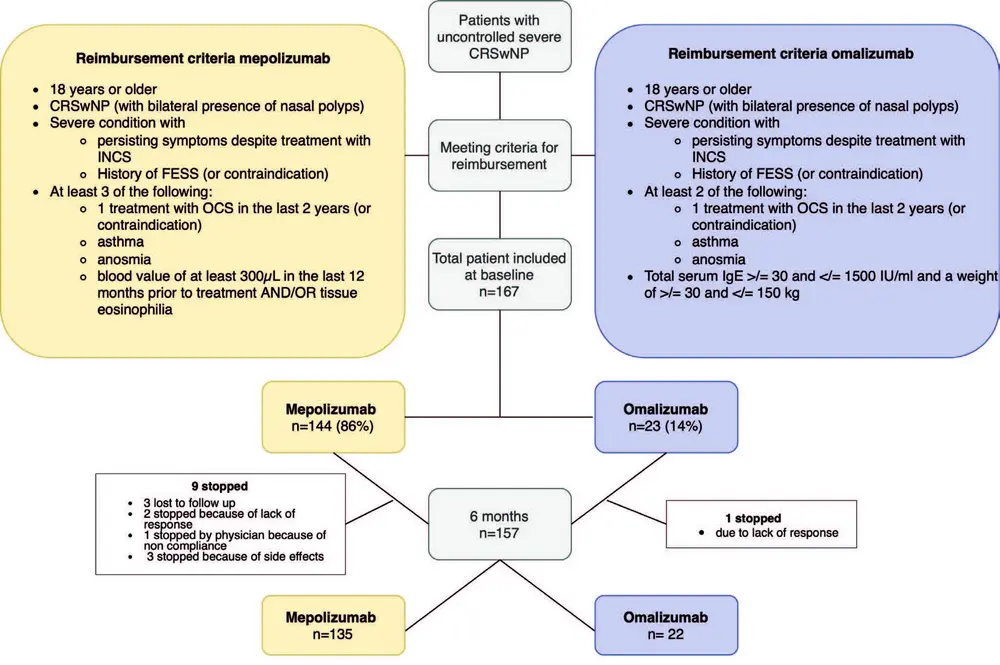
This RWE study was coordinated by A.S.V. and P.H. at UZ Leuven, Belgium, and was performed in 8 national centres, 5 University hospitals (Leuven, Antwerp, Brussels, Liège and Saint-Luc) and 3 non-academic rhinology centres (Aalst, Genk and Bruges). The study was evaluated and approved by the medical ethical committee of the University Hospital of Leuven (S66646). Patients that fulfilled both clinical- and reimbursement criteria (Figure 1) for mepolizumab or omalizumab were included in the trial and followed up prospectively. Patients were excluded if they received another biological treatment for CRSwNP within the 3 months before the start of the study.
Baseline characteristics
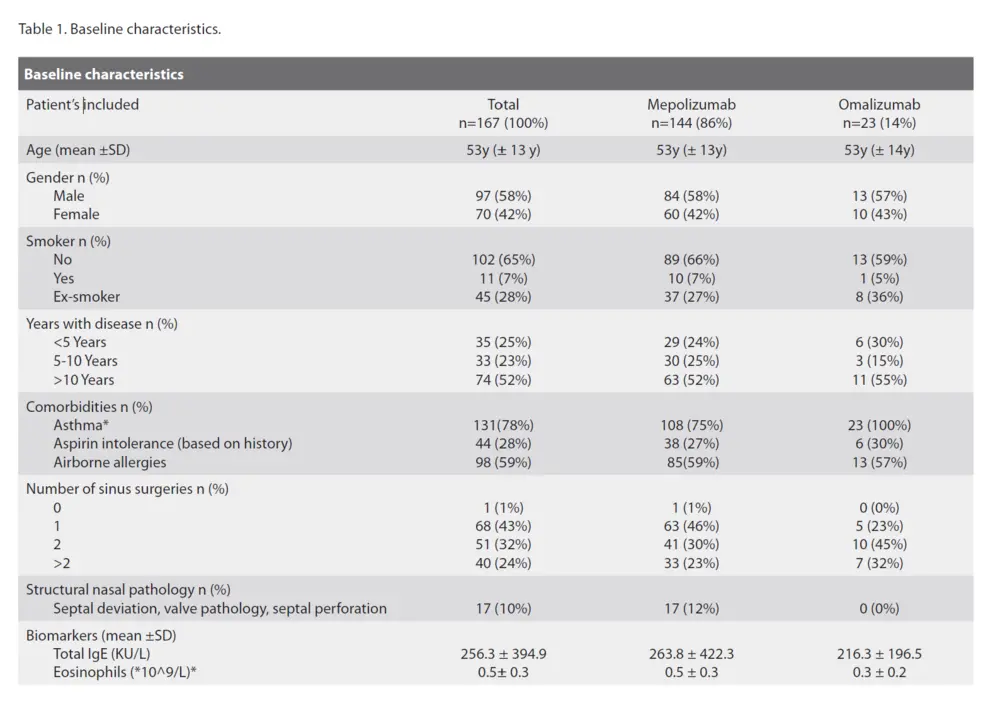
General patient characteristics including age, gender, year of diagnosis of CRSwNP, comorbidities and general health were collected. Patients were asked about their comorbidities, which were verified using the patients’ medical history in their medical record.
Evaluation of patient reported outcomes (PRO’s)
Questionnaires were filled out at the start of treatment and at 4, 12 and 24 weeks of treatment. The questionnaires included questions about the upper airways (Nasal congestion score (NCS), Visual analogue scale (VAS)-score, Sinonasal Outcome test (SNOT) 22-score) and lower airways (asthma control questionnaire (ACQ- 5) and asthma quality of life questionnaire (AQLQ)).
Evaluation of nasal polyp score (NPS)
The nasal polyp size was scored via the NPS, ranging from 0 (no polyp) to 4 (nasal polyps reaching the bottom of the nasal cavity) on each side using nasal endoscopy, as reported before (23).
Evaluation of therapeutic response (TRE) and disease state The therapeutic response to a biological was measured using the following 5 criteria in accordance with the EUFOREA/EPOS criteria (21): 1) reduction in NPS, 2) reduced need for OCS/salvage surgery, 3) improved sense of smell, 4) improved quality of life and 5) reduction of the impact of comorbidities. A good-excellent response was defined as meeting 4 to 5 criteria, a moderate response 2 to 3 criteria and no/poor response 0 to 1 criteria.
The level of control was measured according to the EUFOREA/EPOS criteria for assessment of control in CRSwNP patients at baseline and 3, 12 and 24 weeks after the start of biological therapy. Patients with a VAS-score of ≤ 5 cm for overall sinonasal symptom severity were defined as controlled. The overall symptom severity was based on the individual VAS-scores for nasal blockage, rhinorrea, anosmia, post-nasal drip, facial pain and headache (21).
Evaluation of adverse events and treatment adherence
At each outpatient visit, patients were asked whether they experienced any adverse events (AE), whether they were prescribed antibiotics and/or oral corticosteroids or whether they underwent a revision ESS. In addition, patients were checked for adherence to the treatment scheme with biologicals, with nasal corticosteroid and nasal irrigations as standard of care.
Data analysis
Statistical analyses were performed with GraphPad Prism VI for Macintosh Version 8.4.3 (GraphPad Software Inc., San Diego, CA, USA). Differences in baseline characteristics between the two biologicals were calculated using Fisher’s exact test for categorical variables and unpaired t-test for continuous variables. Differences between nasal polyp score and the different PRO’s at different timepoints were calculated using mixed effect analysis with Tukey’s multiple comparisons test. Differences between the VAS-scores at 24 weeks and baseline were calculated using paired T-test or Wilcoxon signed rank test depending on normality. Values will be considered statistically significant if p < 0.05.
Results
Baseline characteristics
167 CRSwNP patients were included at baseline of which, 144 (86 %) were treated with mepolizumab and 23 (14 %) with omalizumab, on top of nasal rinses and nasal corticosteroid therapy (Figure 1). The mean age was 53 years in both groups. There were almost 10% more males than females in our cohort, an average disease duration of more than 10 years, and the majority of patients had undergone one or two ESS in the past. All of the patients on omalizumab had comorbid asthma compared to 75 % of patients on mepolizumab. This difference can be attributed to the reimbursement criteria for omalizumab in Belgium in 2022 and 2023 with comorbid asthma being a criterium, which changed in November 2023. Patients in the mepolizumab group had a significant higher blood eosinophil count compared to the omalizumab group. Other parameters are listed in Table1.
Evaluation of main clinical parameters
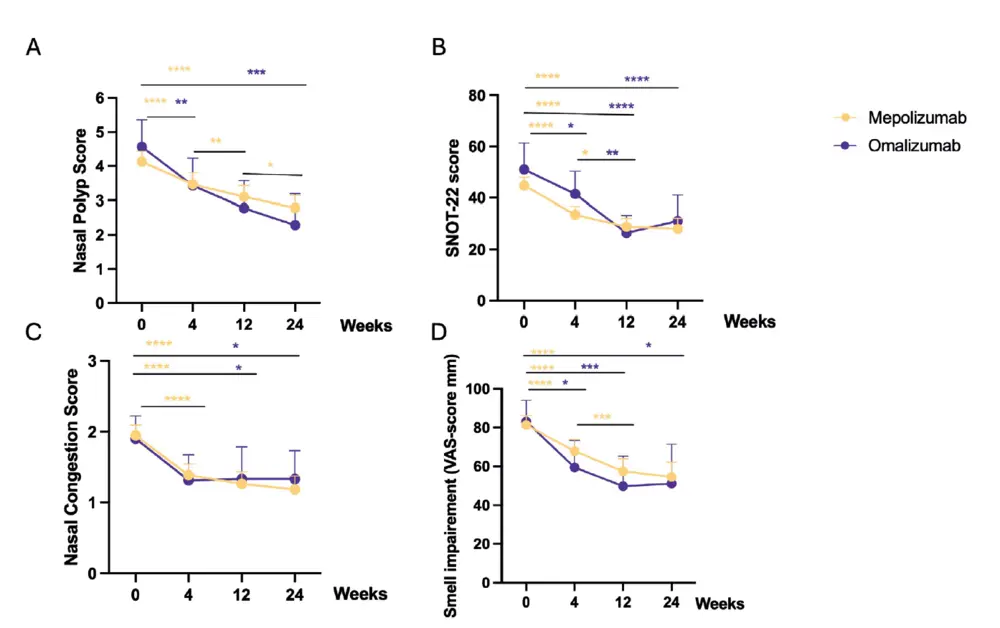
A significant difference was seen between baseline and 24 weeks of treatment for both biologicals on all main clinical parameters: NPS, SNOT-22, loss of smell and NCS. The mean NPS at baseline was 4.1 (CI 4.4-3.8) for mepolizumab
and 4.5 (CI 5.4-3.8) for omalizumab and dropped with a mean difference of 1.3 (CI -1.7 to -0.97) and 2.2 (CI -3.6 to -1.0) points, respectively (Figure 2A). The mean SNOT-22 score at baseline was 44.8 (CI 48.0-41.5) and 51.0 (CI 61.4-40.7) and dropped with a mean of -17.2 (CI -21.1 to -13.3) and -18.9 (CI -28.6 to -9.2) for mepolizumab and omalizumab, respectively (Figure 2B). The nasal congestion score dropped from 2 (IQR 3-1) at baseline to 1 (IQR 2-0) at 4 weeks, and remain stable around this level throughout the trial, representing only mild symptoms (Figure 2C). Moreover, there was a significant reduction in VAS-scores for anosmia between baseline and 24 weeks with a mean reduction of -27 (CI -36 to -18) mm for mepolizumab and -32 (CI -57 to -7) mm for omalizumab. Although, the mean VAS-score for anosmia at 24 weeks remained high at 55 (CI 62-47) for mepolizumab and 51 (CI 71-31) for omalizumab (Figure 2D). Between 12 and 24 weeks, the NPS decreased significantly further in the mepolizumab but not in the omalizumab group. This reduction was not seen for SNOT-22, NCS and smell impairment scores (Figure 2A-D).
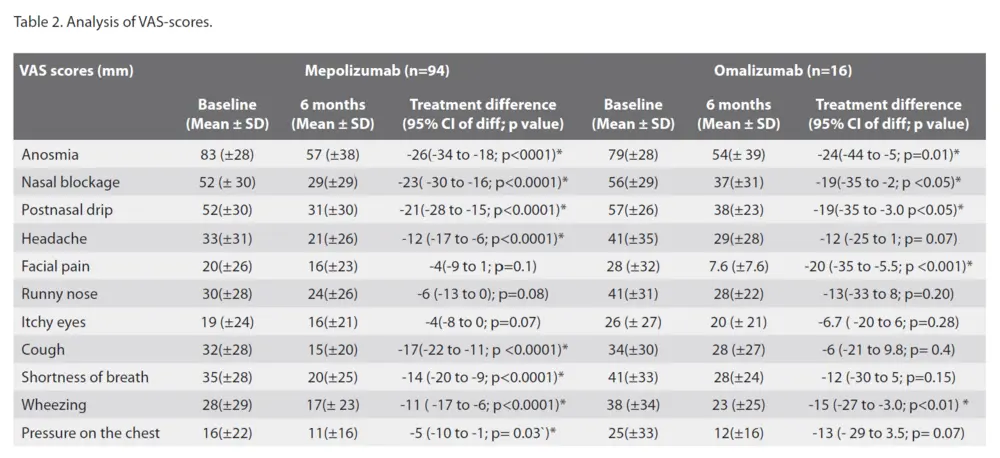
Looking at the VAS-scores for the different sinonasal symptoms, the largest reduction in VAS-scores was for smell impairment, as reported above. Other important symptoms were nasal blockage and postnasal drip. The nasal blockage VAS-scores reduced with a mean of -23 (CI -30 to -16) mm for mepolizumab and -19 (CI -35 to -2) mm for omalizumab. For postnasal drip the VAS-scores reduced with a mean of -21 (CI -28 to -15) mm for mepolizumab and -19 (CI -35 to -3.0) mm for omalizumab. Other symptoms such as headache and facial pain were already scored quite low at baseline, with VAS-score for headaches only significantly improving in mepolizumab with -12(CI -17 to -6) mm and VAS-score for facial pain in omalizumab -20 (CI -35 to -5.5) mm (Table 2).
Evaluation of lower airway symptoms
This study also evaluated the effects of the biologicals on lower airway symptoms in CRSwNP, since 80% of our patient
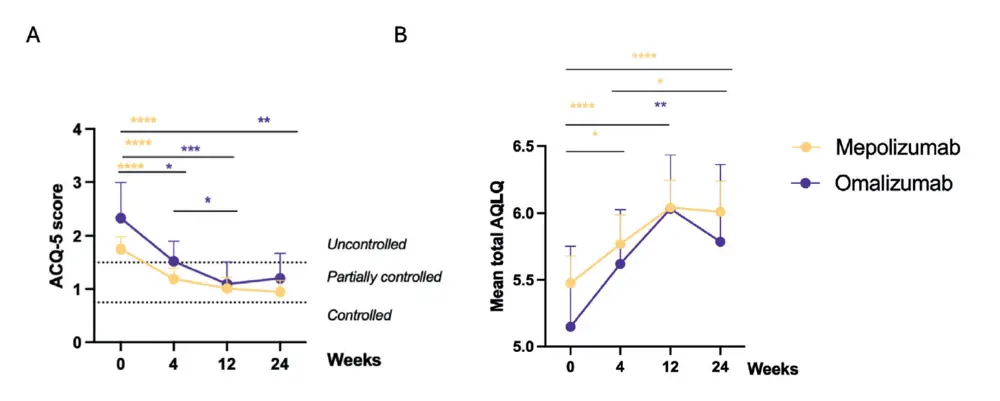
cohort had comorbid asthma. A significant effect was observed on patient reported control of asthma as measured with the ACQ-5 score (Figure 3A). Most of our patients suffered from an uncontrolled asthma at baseline with a mean ACQ-5 score of 1.8 (CI 2.0-1.5) in the mepolizumab group and 2.3 (CI 3.0-1.7) in the omalizumab group. Under biotherapy, the ACQ-5 score generally improved to partially controlled asthma at 24 weeks, with a mean improvement of -0.8 (CI -1.1 to -0.5) for mepolizumab and -1.1 (CI 2.0 to -0.3) for omalizumab. (Figure 3A) Looking at the quality of life, measured with the AQLQ score, a significant improvement for mepolizumab at 24 weeks with a mean improvement of 0.5 (CI 0,3 to 0,8) was seen (Figure 3B). A significant improvement for both biologicals was seen on the wheezing VAS-score, with a mean reduction of -11 (CI -17 to -6) mm for mepolizumab and -15 (CI -27 to-3) mm for omalizumab (Table 2).
Therapeutic response evaluation (TRE) and disease state at 24 weeks
At 24 weeks, the prolongation of the treatment is decided based on the TRE on the one hand and the national reimbursement criteria on the other hand. In 81% of patients on mepolizumab and 74% on omalizumab the treatment was continued beyond 24 weeks (Figure 4A). 45% of patients on mepolizumab and 47% of patient on omalizumab had an excellent TRE according to the EUFOREA/EPOS criteria with only 14% of mepolizumab and 12% of omalizumab patients having no/poor response to a biological (Figure 4B). Although almost 50% of patients had an excellent response to their biological at 24 weeks, control was only reached in 34% of mepolizumab and 35% of omalizumab patients (Figure 4D). Amongst those with an excellent TRE, 65% on mepolizumab and 75% on omalizumab met the criteria of being controlled (Figure 4C). In the patient cohort that continued their biological therapy, control was reached in 41% and 46% of patients on mepolizumab and omalizumab, respectively. Lastly, in the group that stopped their biological the main reason was lack of response, only three patients stopped because of side effects (shingles and/or musculoskeletal pain) and one patient was stopped by the physician because of a lack of compliance (Figure 1). 50 % of patients who stopped omalizumab and 36% of patients who mepolizumab were switched to another biological.
Treatment adherence and safety
No severe adverse events were reported during the initiation and course of the biological therapy. Most patients self-administered their injections at home after two or three injections in the clinic under medical supervision. Self-reported treatment adherence was good with only 5% of patients reporting to have missed an injection, primarily due to external factors such as limited availability of the biological at the pharmacy or expiration of reimbursement coverage before all syringes could be obtained. As mentioned before, only three patients stopped their treatment early because of side effects (Figure 1).
Discussion
This registry in eight rhinology centres in Belgium includes 167 patients with severe CRSwNP and reports on the RWE of omalizumab and mepolizumab in patients with CRSwNP meeting national reimbursement criteria. This RWE study confirms the efficacy of both biologicals, as reported in DBRCT, with a significant improvement on all primary outcome parameters at 12 and 24 weeks of therapy. Disease control was achieved in one third of patients in both groups at 24 weeks.
When comparing these Belgian real-world data with the reported DBRCT trials (7,9,10,12) several observations can be made. Firstly, the baseline SNOT-22 score, and the mean improvement observed in our study (-17.2 to -18.9) were comparable to the average reduction reported in both DBRCT of omalizumab and mepolizumab (-9.40 to -16.2), indicating comparable efficacy in reducing disease burden (12). NCS reduction in this trial at 24 weeks was similar to the one reported in the omalizumab phase III trials (7), as was the case with VAS-scores for loss of smell, with the reductions observed here being similar as in the mepolizumab phase III trials (9). Interestingly, the baseline NPS in the current cohort was lower (4.1–4.5) than in DBRCTs, where a TNPS of 5 or more was an inclusion criterium for randomization. In spite of the lower baseline NPS in this registry, the standard mean difference at 24 weeks in NPS reported here was larger 1.3 (CI -1.7 to -0.97) and 2.2 (CI -3.6 to -1.0), compared to the DBRCTs -0.85 (CI -1.06 to -0.64). This observation might be explained by the inclusion of less severe patients in our RWE than in the RDBCT, or by the unblinded scoring of NPS in the RWE compared to RDBCT. Haxel et al. (17) also reported a similar trend of more pronounced clinical improvements in their RWE data compared to the mean changes in the phase III data. They suggested a better type II inflammatory disease selection in real-life settings, where type II-associated co-morbidities, such as asthma, are more prevalent than in RDBCTs. Indeed, in our cohort 80% has comorbid asthma, which in line with other RWE studies (15,17,24,25) but is notably higher than the 60-70% reported in phase III trials (7,9).
Interestingly, major effects of biological therapy were seen at 12 weeks of therapy without significant further improvement
in PRO’s such as NCS, SNOT-22 score, AQLQ or ACQ5 -score between 12 and 24 weeks. Of note, the NPS did further decrease between 12 and 24 weeks of therapy in the mepolizumab group only, without the associated impact on subjective parameters. This observation warrants some context. Firstly, NPS does not directly correlate with patient relevant outcome parameters like smell loss, congestion or SNOT scores (26). Secondly, the lack of additional benefit of omalizumab after 12 weeks does not exclude an additional benefit beyond 24 weeks of therapy and might be a consequence of the limited sample size compared to the mepolizumab group. An indirect comparison study in the past (10), already hinted at a possible plateau effect after 14 weeks of therapy with limited differences seen between 16 and
24 weeks of treatment in the clinical trials with both omalizumab and mepolizumab. This phenomenon was further confirmed by several other RWE studies focusing on dupilumab and/or omalizumab (16,17). We now confirm this finding in both omalizumab and mepolizumab patients. We can only speculate on a further decrease in both NPS and/or patient relevant parameters beyond 24 weeks of therapy. However, the study of Cavaliere et al. showed a significant improvement between 24 weeks and 52 weeks on NPS, SNOT-22 and loss of smell VAS-scores in patient treated with mepolizumab.
Comparing our TRE data with other RWE studies of biologicals, we can primarily draw comparisons with dupilumab registries such as the Italian registry of De Corso et al. (14), the Dutch cohort of Van der Lans et al. (15) and the German study of Haxel et al. (17), which was the only one to analyse TRE on a population that comprised of both dupilumab as well as omalizumab patients (14,15,17).
Strikingly, more patients in the Italian cohort (57.7%) and the German cohort (56%) reached an excellent therapeutic response compared to our cohort (45-47%) and the study from Van der Lans et al. (40.7%). Moreover, a higher percentage of nonresponders was seen in our patient cohort (12-14%) compared to the cohort from De Corso et al. (1.2-1.9%), Van der Lans et al. (3.7%) and Haxel et al. (1%) (14,15,17). However, it may well be that longer treatment, or follow-up might reveal a higher percentage of patients with good to excellent therapeutic response than currently reported at 24 weeks. Caution is warranted when interpreting results from different registries as the historic criteria to define a therapeutic response slightly differ between registries, and reimbursement criteria differ between countries (1,14,15,17).
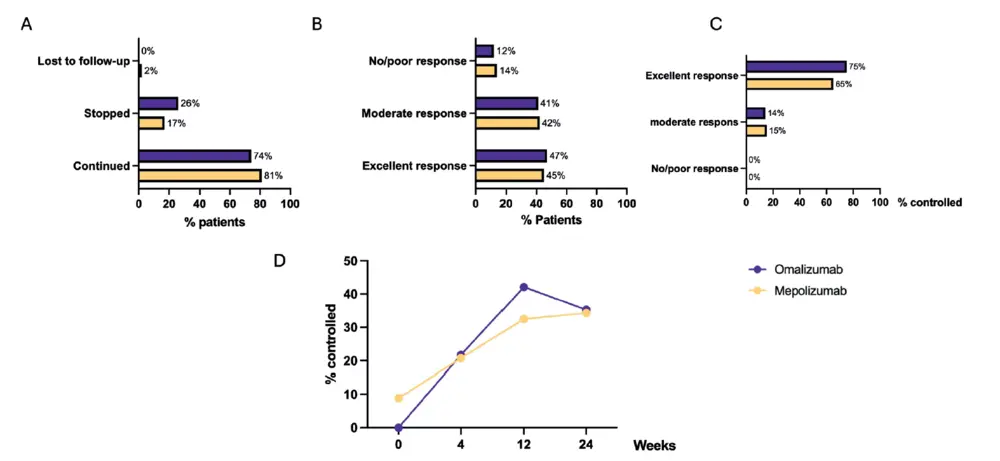
As mentioned before, this report is made to include the newly consented definition of control in the real-world context of a
Belgian registry (21). It is striking that, although nearly 50% of patients treated with omalizumab or mepolizumab showed an excellent response to their biological according to the EUFOREA/EPOS criteria (21), only about one-third achieved disease control. This discrepancy can be largely attributed to the fact that the mean anosmia VAS-score at 24 weeks remained rather high around 54 out of 100, despite a significant reduction in loss of smell VAS-score. This prevented many patients from meeting a disease state of control, as defined by a lack of bothersome symptoms with VAS-scores of < 50 mm. Several factors should be considered when interpreting this data: most patients initially presented with a high VAS-score (i.e. 8/10). Despite anosmia VAS-scores remaining high at 24 weeks, both treatment groups
showed a significant average reduction of 2 points. Additionally, most of our patients had 1 or 2 sinus surgeries in the past, which may have contributed to the persistent loss of sense of smell. This factor could have potentially influenced the anosmia VAS scores. For this reason, the effectiveness of biologics is analysed not solely on the sense of smell but through several other clinical outcome parameters such as the effects on SNOT-22 and NPS. We acknowledge that semi-objective smell testing would have given additional information as to the extent of the anosmia. However, as this was not part of the standard of care in most hospitals, it was not incorporated into this observational trial.
As more long-term data will become available, it will be interesting to observe how the level of control will change in these patients and if some of them are on the path to remission. In our registry, we do not seem to observe differences in efficacy nor control state between mepolizumab and omalizumab at 12 and 24 weeks. However, this might be influenced by the smaller cohort of patients on omalizumab (23 patients) compared to the mepolizumab cohort (144 patients), reflecting the stricter reimbursement criteria for omalizumab in the first year of reimbursement in Belgium, which seemed an important determinant in the choice of a biological in our Belgian cohort. Additionally, the lack of a need for dose calculations or multiple injection schemes with mepolizumab may have contributed to its perceived ease of prescription.
Lastly, patient adherence to their biological was notably high, with 95% of patients not missing a single injection, and dropout rates were as low as 2%. When missed injections occurred, they were primarily due to external factors such as limited availability of the biological at the pharmacy or expiration of reimbursement coverage before all syringes could be obtained. This high adherence rate is likely attributable to the relatively rapid and significant clinical improvements experienced by patients receiving biologicals, in contrast to other treatments such as nasal rinses and/or intranasal corticosteroids and may potentially also be contributed to the high cost of the therapy. Consistent with findings from other clinical trials and RWE studies, the safety profile was good with only minor side effects reported. Specifically, only three patients out of the 167 discontinued treatment due to potential biological-related adverse effects, such as shingles or musculoskeletal pain (13,27).
Conclusion
Six months of treatment with either mepolizumab or omalizumab significantly improved patient-reported outcomes,
with major effects seen at 12 weeks and further reduction in NPS between 12 and 24 weeks in the mepolizumab treated
group. Nearly half of the included patients showed an excellent therapeutic response to their biological with one third reaching control by week 24. Additional follow-up beyond six months is warranted for the evaluation of additional effects on the one hand and progress to remission on the other hand.
Acknowledgements
We want to thank all the principal investigators and sub-investigators of the RELIBIO-trial for their efforts.
Authorship contribution
PH: conceptualization, methodology, review and editing, supervision; ASV: Data Collection, formal analysis, writing, original draft preparation, review and editing; LB: data collection, formal analysis; EB: data collection, review and editing; SH, VH, MJ, WL, FR, KS, LVG, OV, BV, AV: data collection, review and editing; KM: review and editing; All authors have read and agreed to the published version of the manuscript.
Conflict of interest
All authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
There was no specific funding/support for this project.
References
1. Fokkens W, Viskens AS, Backer V, et al. EPOS/EUFOREA update on indication and evaluation of Biologics in Chronic Rhinosinusitis with Nasal Polyps 2023. Rhinology. 2023. 61(3):194-202.
2. Fokkens WJ, Lund VJ, Hopkins C, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology. 2020;58(Suppl S29):1-464.
3. Orlandi RR, Kingdom TT, Smith TL, et al. International consensus statement on allergy and rhinology: rhinosinusitis 2021. Int Forum Allergy Rhinol. 2021;11(3):213-739.
4. Hellings PW, Fokkens WJ, Orlandi R, et al. The EUFOREA pocket guide for chronic rhinosinusitis. Rhinology. 2023;61(1):85-89.
5. European commission. Union Register of medicinal products [Internet]. Available from: https://ec.europa.eu/health/documents/community-register/html/.
6. U.S. Food & drug administration. FDAApproved Drugs [Internet]. Available from: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm.
7. Gevaert P, Omachi TA, Corren J, et al. Efficacy and safety of omalizumab in nasal polyposis: 2 randomized phase 3 trials. J Allergy Clin Immunol. 2020;146(3):595-605.
8. Bachert C, Han JK, Desrosiers M, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet. 2019;394(10209):1638-1650.
9. Han JK, Bachert C, Fokkens W, et al. Mepolizumab for chronic rhinosinusitis with nasal polyps (SYNAPSE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Resp Med. 2021;9(10):1141-1153.
10. Hellings PW, Verhoeven E, Fokkens WJ. State-of-the-art overview on biological treatment for CRSwNP. Rhinology. 2021;59(2):151-163.
11. Peters AT, Han JK, Hellings P, et al. Indirect treatment comparison of biologics in chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol Pract. 2021;9(6):2461-2471.
12. Cai S, Xu S, Lou H, Zhang L. Comparison of different biologics for treating chronic rhinosinusitis with nasal polyps: a network analysis. J Allergy Clin Immunol Pract. 2022;10(7):1876-1886.
13. Oykhman P, Paramo FA, Bousquet J, Kennedy DW, Brignardello-Petersen R, Chu DK. Comparative efficacy and safety of monoclonal antibodies and aspirin desensitization for chronic rhinosinusitis with nasal polyposis: A systematic review and network meta-analysis. J Allergy Clin Immunol. 2022;149(4):1286-1295.
14. De Corso E, Pasquini E, Trimarchi M, et al. Dupilumab in the treatment of severe uncontrolled chronic rhinosinusitis with nasal polyps (CRSwNP): a multicentric observational Phase IV real-life study (DUPIREAL). Allergy. 2023;78(10):2669-2283.
15. Lans RJL vd, Fokkens WJ, Adriaensen GFJPM, Hoven DR, Drubbel JJ, Reitsma S. Real-life
observational cohort verifies high efficacy of dupilumab for chronic rhinosinusitis with nasal polyps. Allergy. 2022;77(2):670-674.
16. Kilty SJ, Lasso A. Canadian real-world study of access and clinical results using dupilumab for chronic rhinosinusitis with polyps. J Otolaryngol Head Neck Surg. 2022;51(1):17.
17. Haxel BR, Hummel T, Fruth K, et al. Realworld-effectiveness of biological treatment for severe chronic rhinosinusitis with nasal polyps. Rhinology. 2022;60(6):435-443.
18. Domínguez-Sosa MS, Cabrera-Ramírez MS, Marrero-Ramos MDC, et al. Real-life effectiveness of mepolizumab in refractory chronic rhinosinusitis with nasal polyps. Biomedicines. 2023;11(2):485.
19. Lombardo N, Piazzetta GL, Lobello N, et al. Real-life effects of omalizumab on chronic rhinosinusitis with nasal polyposis. J Pers Med. 2023;14(1): 3.
20. Belgisch centrum voor farmacotherapeutische informatie. Gecommentarieerd geneesmiddelenrepertorium [Internet]. Available from: https://www.bcfi.be/nl/
21. Fokkens WJ, De Corso E, Backer V, et al. EPOS2020/EUFOREA expert opinion on defining disease states and therapeutic goals in CRSwNP. Rhinology. 2024;62(3):287-298.
22. Xu X, Reitsma S, Wang Y, Fokkens WJ. Updates in biologic therapy for chronic rhinosinusitis with nasal polyps and NSAIDexacerbated respiratory disease. Allergy. 2022. 77(12):3593-3605.
23. Gevaert P, De Craemer J, Bachert C, et al. European Academy of Allergy and Clinical Immunology position paper on endoscopic scoring of nasal polyposis. Allergy. 2023;78(4):912-922.
24. Cavaliere C, Loperfido A, Ciofalo A, et al. Real-life evidence of mepolizumab treatment in chronic rhinosinusitis with nasal polyps: a multicentric study. J Clin Med. 2024;13(12): 3575.
25. Kiricsi A, Bella Z, Kraxner H, et al. Real-life effectiveness of dupilumab in chronic rhinosinusitis with nasal polyps. Results from eight Hungarian centres with 12-month follow-up. Rhinology. 2024;62(4):410-420.
26. Jeong S, Chen T, Nguyen S, Edwards T, Schlosser R. Correlation of polyp grading scales with patient symptom scores and olfaction in chronic rhinosinusitis: a systematic review and meta-analysis. Rhinology. 2022;60;5: 322-334.
27. Dorling M, Hernaiz-Leonardo JC, Pascual A, Janjua A, Thamboo A, Javer A. Real-world adverse events after type 2 biologic use in chronic rhinosinusitis with nasal polyps. Laryngoscope. 2024;134(7):3054-3059.
Peter W. Hellings
Herestraat 49
3000 Leuven
Belgium
Tel: +32486948703
E-mail: peter.hellings@kuleuven.be
An-Sofie Viskens1,2, Laura Bollens3, Elien Borgers4, Stijn Halewyck5, Valérie Hox6, Mark Jorissen4,7, Winde Lemmens8, Florence Rogister9, Kato Speleman10, Laura Van Gerven1,4,7, Olivier Vanderveken2,11, Benedicte Verhaeghe12, Anneclaire Vroegop2,11, Katleen Martens1, Peter W. Hellings1,4,13
1 KU Leuven Department of Microbiology, Immunology and Transplantation, Allergy and Clinical Immunology Research Unit, Leuven, Belgium
2 Faculty of Medicine and Health Sciences, University of Antwerp, Antwerp, Belgium
3 Faculty of Medicine, KU Leuven, Leuven, Belgium
4 Department of Otorhinolaryngology-Head and Neck Surgery, UZ Leuven, Leuven, Belgium
5 Department of Otorhinolaryngology, University Hospital Brussels, Jette, Belgium
6 Department of Otorhinolaryngology, University Hospital Saint-Luc, Brussels, Belgium
7 Laboratory of Experimental Otorhinolaryngology, Department of Neurosciences, KU Leuven, Leuven, Belgium
8 Department of Otorhinolaryngology, hospital Oost-Limburg, Genk, Belgium
9 Department of otorhinolaryngology, CHU de Liège, Belgium
10 Department of Otorhinolaryngology, General hospital Sint-Jan Bruges, Bruges, Belgium
11 Department of Ear-Nose-Throat, Head and Neck Surgery, Antwerp University Hospital, Edegem, Belgium
12 Department of Otorhinolaryngology, Onze Lieve Vrouw hospital Aalst, Belgium
13 Upper Airways Research Laboratory, University of Ghent, Ghent, Belgium
Rhinology 63: 3, 316 - 324, 2025 https://doi.org/10.4193/Rhin24.463
Received for publication: October 30 , 2024
Accepted: March 8, 2025
Associate Editor: Sietze Reitsma

